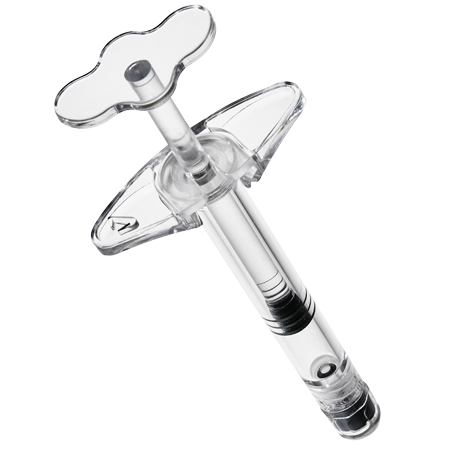
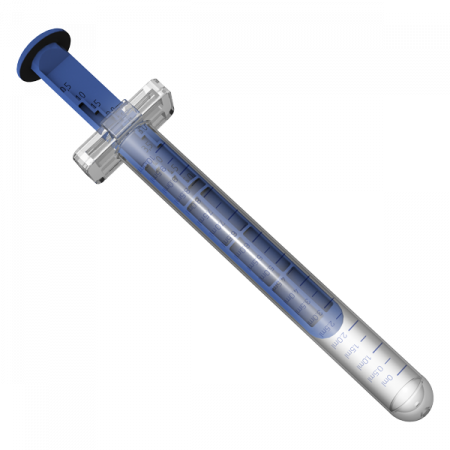
Our 3 main product lines

Choosing our products means enhancing your own
Our trademarked products are not simply “drug delivery system” or “primary Pharmaceutical packaging“.
They are designed and produced to enhance our customers’ products so that they stand out even more.
In fact, all of our devices:
- are conceived, designed and manufactured to be robust, functional and reliable;
- implement new technical solutions and have a significant innovative content that responds to issues and/or needs never faced before;
- are highly customisable;
- are subjected to thorough checks that ensure their absolute quality.
Our strengths: Quality, Speed and flexibility
We are able to maintain high quality also with fast deliveries. Whether they are small batches, e.g. for validation tests, or large ones, this also applies during the production of products customised or to specification. In fact:
- we always perform a production feasibility analysis during the product development stage. This speeds up times and prevents errors. we employ versatile automatic machines that can be quickly fitted to switch from the production of one device to another, in order to be fast and flexible;
- our staff has been specifically trained. Quality controls are extremely thorough;
- our staff has been specifically trained. Quality controls are extremely thorough;
- we internally design and manufacture both tools and automatic assembly machines;
- we complete the offer with a secondary and tertiary packaging service.


Customer needs come first:
We listen to our customers, physicians and end consumers.
This allows us to know:
- how to support our customers efficiently;
- which device to develop;
- what contribution we can make to the market and how we can improve the life of end users ;
- which problem or need has yet to be answered.
Our continuous research and attention to unmet needs allows us to develop devices that greatly differ from our competition and that make our customer’s product stand out from their competitors.
The needs of both our prospects and our customers lead us to continuously develop new technical solutions and new ideas, anticipating market trends.
Il Sistema Qualità alla base delle fasi di sviluppo dei devices
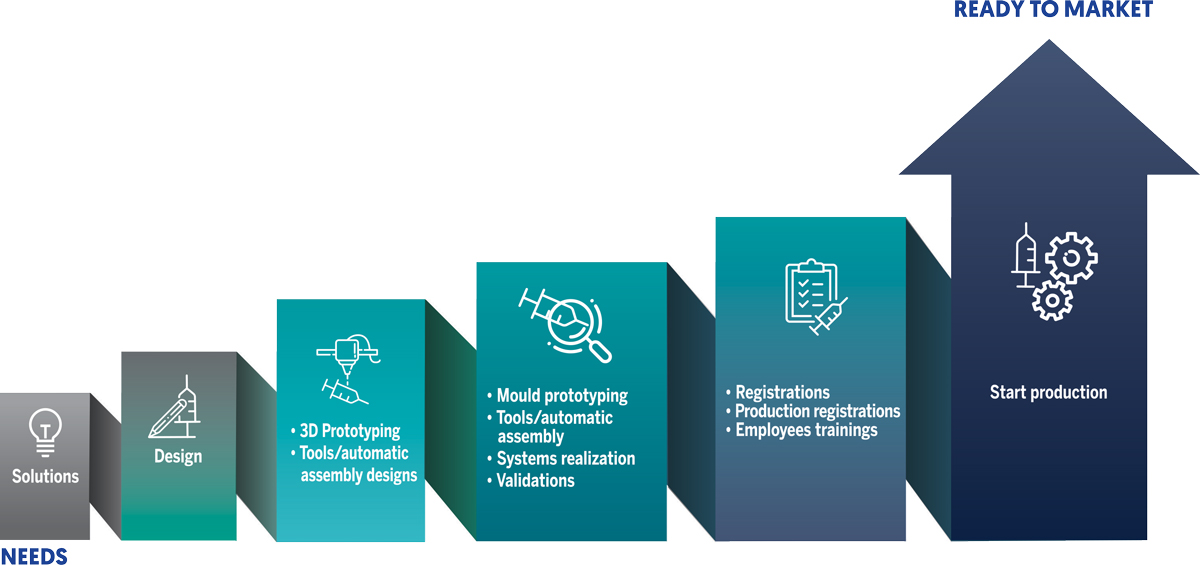
Il Sistema Qualità alla base delle fasi di sviluppo dei devices
1
Partendo dagli obiettivi e richieste del nostro cliente, proponiamo alcune soluzioni e insieme si sceglie la migliore;
2
Realizziamo disegni 3D e 2D, e ci confrontiamo sia con il reparto di ingegneria che con i tecnici dello stampaggio su criticità legate alla produzione e realizzazione di macchine e tools di assemblaggio;
3
Realizziamo prototipi in stampa 3D. Verifichiamo usability, aspetto estetico, dimensioni di ingombro;
4
Produciamo gli stampi pilota. I primi pezzi del devices prodotti tramite injection moulding, vengono attentamente e scrupolosamente testati, e misurati al centesimo, mediante tecnologia 4K e reverse engineering, per verificarne la rispondenza al progetto iniziale, calcolarne i ritiri di materiale, verificare eventuali difetti, al fine di ottimizzare gli stampi pilota. In genere in 1-2 passaggi gli stampi pilota sono ottimizzati. Contestualmente si progettano i tools per l’assemblaggio o i sistemi automatici per l’assemblaggio, a seconda dei volumi di produzione previsti;
5
Inseguito all’accettazione iniziano i test di convalida del prodotto, le prove di tipo interne e la stesura della documentazione tecnica. Si avvia la realizzazione degli stampi definitivi e dei tools per l’assemblaggio;
6
Il device viene messo in produzione a prescindere dalla del lotto.
Certifications and quality system
All our production processes are planned and regulated by the “quality assurance department”.
All production workers are trained by the engineering department and by “quality assurance” before the start of production.
The quality system also involves our suppliers, who we audits periodically just as our Customers do to us in compliance with the highest pharmaceutical standards.
Our products are currently distributed in the USA, Europe and China.
We are ISO 13485:2016 certified
Internal production in ISO 7 environment